
Only latent TB test for children 2 and above
The T-SPOT.TB test is the only IGRA, or TB blood test, cleared by the United States Food and Drug Administration (FDA) for your patients two and older, including your BCG-vaccinated patients, healthy patients, and patients with compromised immune systems.1
TB guidelines support IGRA use for children
Only IGRA cleared by the US FDA for ages two and up1
When it comes to children, you know that not all diagnostic tests have the same accuracy in pediatric populations as they do in adults. Fortunately, the T-SPOT.TB test is reliable for not only the healthy children you test, but also for the children that have medical issues such as diabetes, are on a corticosteroid, have rheumatological, gastrointestinal, dermatological issues, renal issues, HIV, and any other medical diagnosis that suppresses their immune system.
Did you know?
The T-SPOT.TB test is also the only interferon-gamma release assay (IGRA) without a warning in its package insert for screening immunocompromised patients.1,2
-
Easy1
The younger the patient, the smaller the blood sample
-
Accurate1
The TB test with the highest reliability, with sensitivity at 98.9% and specificity at 100%, and no cross-reaction with the BCG-vaccine
-
Accessible
Available in Asia-Pacific region

A moment of truth
As a healthcare provider, your goal is to offer your pediatric patients a reliable tuberculosis (TB) blood test. The T-SPOT.TB test is that test. By counting the cells, it mitigates the risk that low cell count will impact patient results.3 Cells are washed to prevent interference from substances or contaminants and then counted to ensure that a standard number of cells, 1 million, are used in the assay.1 You need an answer you can trust. An accurate TB test along with your clinical evaluation of the patient will give you that answer, and a moment of truth you can count on.
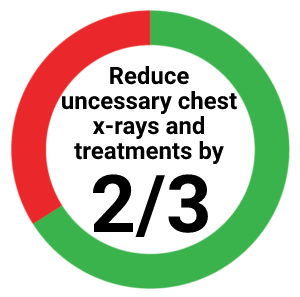
Reduce unnecessary return visits and treatments
- In a study of > 3,600 children under the age of 15, switching from the tuberculin skin test (TST) to an IGRA could reduce unnecessary chest x-rays and treatments by up to two-thirds in non-US born children.4
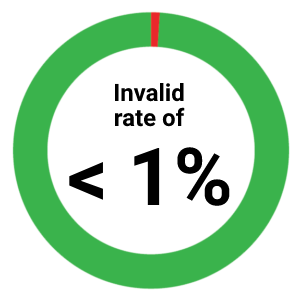
- In study of over > 645,000 samples, the T-SPOT.TB test had an invalid rate of < 1%.5
1 tube, 1 visit and low blood volume requirements1
- Children 10 years old and over: 1 sodium or lithium heparin 6 mL tube
- Children > 2 or < 10 years old: 1 sodium or lithium heparin 4 mL tube
- Children up to 2 years old: 1 sodium or lithium heparin 2 mL pediatric tube*
Did you know?
Samples can be drawn using a butterfly needle without the use of a purge tube. Many butterfly needles have the same needle gauge as a TST6.
Guidelines support IGRA testing
| Guideline | Recommendation |
| WHO Latent Tuberculosis Infection (LTBI): Updated and consolidated guidelines for programmatic management: 2018 | Screening for LTBI with IGRAs and T-SPOT.TB is approved by WHO.
At-risk pediatric populations include: Children living with HIV; Children who are close contacts of positive active TB; Children who are part of other clinical risk groups, such as children receiving anti-TNF treatment, on dialysis, and those preparing for an organ or haematological transplant. |
| Ministry of Health (MOH) Singapore, MOH Clinical Practice Guidelines on Prevention, Diagnosis and Management of Tuberculosis: 2016 | T-SPOT.TB is a commerically approved IGRA, approved for use by Singare MOH.
Close contacts screening for LTBI to either use TST or IGRA
|
| Korean Guidelines for Tuberculosis (4th Edition): 2020 | Children aged 5-18 years: Use IGRA alone. Simultaneous use of TST/IGRA is not recommended as an LTBI test method.
BCG Vaccine: All ages, including those under 5 years of age, have been inoculated after 1 year of age or have been inoculated more than 2 times to check for tuberculosis infection only through IGRA without TST. Close contact screening for LTBI to either use TST or IGRA
|
| American Academy of Pediatrics (AAP) 2018 | IGRAs are indicated in ages 2 and up, and preferred in children who are BCG-vaccinated or unlikely to return to have TST read. |
| Infectious Diseases Society of America (IDSA), American Thoracic Society (ATS) and Centers for Disease Control (CDC), 2017 | Ages 5 and up, IGRAs indicated, especially in those who are not high risk for progressing to active TB. |
| CDC Civil Surgeons 2018 | IGRAs required in ages 2 and up:
|