Latent TB Infection (LTBI) Testing in Taiwan
Tuberculosis in Taiwan
Facts and Figures
TB-endemic country with intermediate TB burden
Reduction of TB incidence the last decade.1
Latent TB Infection (LTBI)
Taiwan began its national TB program to implement LTBI treatment for TB contacts in 2008.
TB incidence from 2005 – 2017.2
Reduction of the risk of disease development among TB-infected infants with LTBI treatment.1
HIV-TB co-infection
Low prevalence in Taiwan
Age group with peak HIV-TB co-infection.3
Latent TB Infection (LTBI) Testing
WHO has identified the need to detect, diagnose and treat latent TB infection (LTBI) as a key strategy to end TB. Testing is the first step to treat those who have LTBI to break the cycle of transmission and infection of TB.
But, when 25% of the world’s population have a prior infection of the Mycobacterium TB bacteria and remain asymptomatic4, and when 1 in 10 with LTBI progresses to active TB, ending TB needs to begin with an understanding of prioritising whom to screen5.
Who should get tested for LTBI?
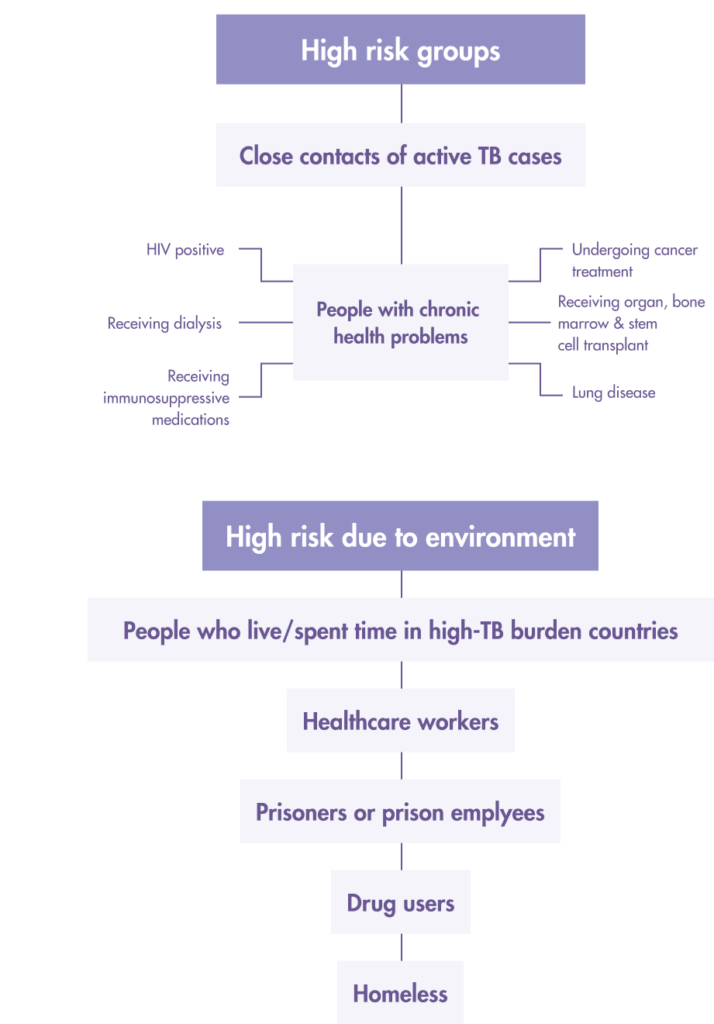
Both the Tuberculin Skin Test (TST) and Interferon-gamma release assays (IGRA) tests, which include the T-SPOT®.TB test are used to aid the detection of latent TB infection in Taiwan.
While the TST seems simple enough to perform and does not require phlebotomy, the clinical and operational limitations of the TST are well documented. Detection of LTBI using the TST is also a challenge to administer and interpret.
TST test and cross-reactivity with the BCG vaccine
Since 1 January 2016, Taiwan Centers for Disease Control (CDC) adjusted the recommended timeframe for BCG vaccination – from 24 hours after birth, or as early as possible, to between 5 – 8 months and no later than 1 years old6. This means that almost all babies are vaccinated BCG. Not all vaccines work equally well, and the BCG has significant limitations in preventing the spread of TB.
TST uses mixture proteins, sometimes referred to as purified protein derivative (PPD), and these protein cross react with BCG and non-tuberculous Mycobacteria (NTM), leading to low specificity in patients with BCG vaccine. Low specificity means higher false positive results especially in children, which leads to overtreatment7.
Limitations with TST that are seen in BCG vaccinated populations are absent when using IGRAs.
IGRAs are an in vitro diagnostic blood test, which detect interferon-gamma released by T cells after exposure to certain TB-specific antigens, as an indirect measure of adaptive cell immunity. The T-SPOT.TB test, which is a type of commercially-available IGRA test, uses TB-specific antigens ESAT-6 and CFP10. These antigens used are important because they are specific for MTB, and not present in the BCG vaccine or non-TB mycobacterium (NTM), limiting false reactions.
TB Guidelines: Taiwan
Taiwan Centers for Disease Control. Guidelines for Diagnosis and Treatment of Tuberculosis. Sixth Edition, 20178.
With the exception of Multi-drug resistance TB (MDR-TB), TB is still classified as the third most infectious disease in Taiwan since 1996. Screening and diagnosis of active and latent TB infection includes the following tests:
1. For active TB
- Clinical inquiry and physical assessment
- Chest X-ray
- Sputum smear
2. For latent TB infection (LTBI)
- TST (Tuberculin/Mantoux) Test:
TST is only used for contacts < 5 years of age or for those who cannot complete the IGRA test. The interpretation of the test to determine LTBI is maintained at 10mm (for those who are BCG-vaccinated). - Interferon Gamma Release Assay (IGRA) blood test:
IGRA test for the detection of TB infection is based on the quantification of peripheral blood IFN-γ levels after activation by TB-specific antigens ESAT-6, CFP-10. There are two types of IGRA tests in use based on the following methods:
– ELISPOT (enzyme-linked immunospot)e.g. T-SPOT.TB
– ELISA (enzyme-linked immune absorbent)
Since 2016, IGRA tests are used for all people > 5 years and older to reduce false positives due to cross reactivity with the BCG vaccine and unnecessary LTBI treatments.
Prioritize Latent TB infection (LTBI) screening for “high risk” people from these two groups:
1. High-risk LTBI groups
- Close/Frequent contact with active TB patients (across all age groups)
- Patients receiving anti-tumor necrosis factor therapy
- Prisons and prison employees
- Healthcare workers
- Indigenous people living in mountainous regions
- Elderly groups in long-term care institutions.
- Homeless
- New residents or migrant workers from countries with high TB burden
2. People with the following underlying health conditions
- Patients receiving anti-tumor necrosis factor therapy
- People living with AIDS
- Intravenous drug users
- Patients with hematological tumors
- Patient who has lung cancer, diabetes, rheumatism
- Dialysis patients
- Patients who are preparing for organ or bone marrow transplantation
Begin TB preventative treatment only when patients are screened and diagnosed with LTBI to avoid incomplete treatment leading to multi-drug resistant TB (MDR-TB).
References
- Taiwan Centers for Disease Control. Recommendations for postponing BCG vaccination. Available from: https://www.cdc.gov.tw/Uploads/f9ddf264-1994-41a0-bc8e-e120e422a9c6.pdf
- Taiwan Centers for Disease Control. (28 March 2019). IT’S TIME! At-risk groups urged to get LTBI screening as Taiwan continues campaign to end TB. Available from: https://www.cdc.gov.tw/En/Bulletin/Detail/cHsOe6RCP5mIwPAOJRDeDw?typeid=158
- Chen YY, Pan SW, Shen HS, Chuang FY, Feng JY, Su WJ. Declining trend in incidence of tuberculosis in adolescents and young adults in Taiwan. Eur Respir J. 2019 May 2;53(5):1801305. doi: 10.1183/13993003.01305-2018. PMID: 31048371.
- Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019 Sep 12;54(3):1900655. doi: 10.1183/13993003.00655-2019. PMID: 31221810.
- Centers for Disease Control and Prevention. (2018). Deciding when to treat latent TB Infection. Available from: https://www.cdc.gov/tb/topic/treatment/decideltbi.htm
- 衛生福利部疾病管制署. (2015). 105年1月1日起卡介苗接種年齡調整為出生滿5個月,建議接種年齡出生滿5-8個月. https://www.cdc.gov.tw/Category/ListContent/3263Cslsb96HH82yOx-T8A?uaid=kxpu7SYN8j_1_-mUMds9Ug
- Spicer KB, Turner J, Wang SH, Koranyi K, Powell DA. Tuberculin skin testing and T-SPOT.TB in internationally adopted children. Pediatric Infectious Diseases J. 2015 Jun;34(6):599-603. doi: 10.1097/INF.0000000000000680. PMID: 25973937.
- 衛生福利部疾病管制署. (2017). 結核病診治指引, 第六版. https://www.cdc.gov.tw/File/Get/FQWRAW1kzwxQSzQKSXj5iw
Why T-SPOT®.TB?
1 small tube. 1 million ways to find the truth¹
Developed by leading diagnostic company Oxford Immunotec, the T-SPOT.TB test is a single visit blood test for tuberculosis designed to reduce assay variability and maximize sensitivity, even in the immunocompromised.²
IGRA technology is based on the release of interferon-gamma secreted by individual effector T cells (both CD4+ and CD8+) after being stimulated by TB-specific antigens. The T-SPOT.TB test uses the enzyme-linked immunospot (ELISPOT) methodology to enumerate M. tuberculosis-sensitized T cells by capturing interferon-gamma in the vicinity of T cells from which it was secreted.
When your test looks for signs of TB infection using T cells, it matters how many T cells you include. The T-SPOT.TB test, unlike other tests, uses the same number each time. And it’s a big number: 250,000 cells per well, four wells per test¹. That’s a million witnesses to infection – and a moment of truth for TB.
How does the T-SPOT.TB test compare to other latent TB tests?
Learn more about the T-SPOT.TB test
LEARN MOREThe T-SPOT.TB test advantages

-
Accurate across patient populations1
- Immunosuppressed
- BCG vaccinated
-
Onl FDA-approved IGRA for children > 2 years2
-
Consistent results3
- Sensitivity: 98.8%
- Specificity: 99.1%
-
High test accuracy around result cut-off due to regulatory approved borderline zone – helping to prevent inappropriate therapy3, 4
Automation for the T-SPOT.TB test 3, 5
It is now possible to automate the T-SPOT.TB test using the CE-marked T-Cell Select™ reagent kit, giving more streamlined workflows, simplified sample logistics and freeing up technician’s time.
For everyone over the age of two
T-SPOT.TB: Only IGRA cleared by the US FDA for ages two and up1
When it comes to children, you know that not all diagnostic tests have the same accuracy in pediatric populations as they do in adults. Fortunately, the T-SPOT.TB test is reliable for not only the healthy children you test, but also for the children that have medical issues such as diabetes or are immunocompromised.

Contact Us
To find out more about T-SPOT.TB contact us:
Taiwan
Genmall Biotechnology Corporation, Ltd.
Adress:6F., No.145, Xinhu 1st Rd., Neihu Dist.
Taipei City 114, Taiwan (R.O.C.)
Email: sales@genmall.com.tw
Phone: 886-2-27960803
FAX: 886-2-27310833
Singapore
Oxford Immunotec Limited (Singapore Branch)
160 Robinson Road #23-03
SBF Center, Singapore 068914
Email: enquiry-RoA@oxfordimmunotec.com
Phone: +65 6246 6861
References
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-V3. Abingdon, UK. September 2020.
- Oxford Immunotec. T-SPOT.TB Package Insert TB-PI-US-0001 V9. Abingdon, UK. February 2021.
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-v3. Abingdon, UK. 2016.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, IGRA Expert Committee, Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010; 59(RR-5:1-25.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American ThoracicSociety/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. Published online December 8, 2016:ciw694. doi:10.1093/cid/ciw694
台灣的結核病況
事實與數據
受結核病中度影響之地方性流行國家
過去十年來的結核病案例數目降低1
潛伏結核感染 (LTBI)
2008年,台灣以治療潛伏結核感染為目標,為結核病接觸者啟動了國家級計畫。
結核病案例自2005至2017年.2
結核病感染嬰兒接受潛伏結核感染(LTBI)治療發病風險.1
愛滋病與結核病合併感染
盛行率低
愛滋病與結核病合併感染的高峰年齡.3
潛伏結核感染(LTBI)應受測對象

台灣使用結核菌素皮內檢測(TST)以及丙型干擾素釋放分析(IGRA,包含T-SPOT®.TB檢測)檢測,來偵測潛伏結核感染。
雖然TST看似執行簡便,且不需要靜脈切開放血,但許多紀錄顯示其在臨床與操作上多有限制。使用TST來偵測潛伏結核感染(LTBI)在施作與判讀結果上均有其難度。
TST檢測以及與卡介苗(BCG)的交叉反應
台灣疾管署(CDC)自2016年1月1日起,把卡介苗的適合接種年齡由出生24小時後(或立即接種),調整為出生滿5-8個月,至遲1歲前完成,意即幾乎所有嬰兒均會接種卡介苗。然而,疫苗效果並非無懈可擊,且卡介苗在防範結核病傳播上有諸多限制。
TST使用蛋白質混合物,或稱純化蛋白質衍生物(PPD)。這些蛋白質會與卡介苗或非結核分枝桿菌(NTM)交叉反應,導致卡介苗接種者特異度降低。特異度降低意味偽陽性增加(特別是兒童),進而導致過度治療。TST使用蛋白質混合物,或稱純化蛋白質衍生物(PPD)。這些蛋白質會與卡介苗或非結核分枝桿菌(NTM)交叉反應,導致卡介苗接種者特異度降低。特異度降低意味偽陽性增加(特別是兒童),進而導致過度治療。
使用IGRAs檢測,就可以避免TST檢測在卡介苗接種人口中面臨的限制。
IGRAs是一種體外血液診斷檢測,偵測T細胞暴露在結核病特定抗原後,所釋放的丙型干擾素。這是利用後天細胞免疫的一種間接檢測方式。T-SPOT.TB檢測是一種市售的IGRA檢測,使用結核病特定抗原ESAT-6與CFP10。這些是結核桿菌特定的抗原,不會出現在卡介苗或非結核分枝桿菌(NTM)中,進而降低錯誤的交叉反應。
結核病指引:
衛生福利部疾病管制署, 結核病診治指引, 第六版, 20177.
除了多重抗藥性結核病 (MDR-TB),結核病自 1996 年以來仍被列為台灣第三大傳染病。 開放式和潛伏性結核病感染的篩查和診斷包括以下測試:
1. 透過下列檢驗確認是否為開放式結核病例
- 臨床症狀詢問、身體評估
- 胸腔X光
- 痰液抹片
2. 透過下列檢驗確認是否為潛伏性結核病例:
- TST結核菌素皮內檢測(芒圖氏檢測):僅用在未滿 5 歲 的 接 觸 者 或 極 少 數 無 法 完 成 IGRA 檢驗的民眾,其判讀標準維持在 10mm(接種過卡介苗者)。
- 丙型干擾素釋放分析(IGRA)結核病血液檢測:使用IGRA檢測來偵測結核病感染的原理,是受結核病特定抗原(ESAT-6, CFP-10)激發之後,測量周邊血液丙型干擾素濃度,根據下列方式,有兩種不同的IGRA檢測:
– ELISPOT(酶聯免疫斑點法),例如:T-SPOT.TB
– ELISA(酶聯免疫吸附分析法)
從2016 年起,凡是五 歲以上的接觸者,都優先使用 IGRA,以減少因大量接種卡介苗引起的偽陽性和不必要的 LTBI 治療。
優先篩檢下列兩類「高風險」潛伏性結核感染族群:
1. 高風險潛伏性結核感染組群:
- 全年齡層的結核病接觸者
- 接受抗腫瘤壞死因子治療的病人
- 矯正機關收容人及僱員
- 醫療從業人員、
- 原住民
- 長照機構老人族群
- 遊民
- 來自結核高負擔國家新住民或移工
2. 符合下列健康狀況者:
- 接受抗腫瘤壞死因子治療的病人
- 愛滋病患者
- 靜脈注射藥癮者
- 血液腫瘤患者
- 肺癌、糖尿病、風濕病患者
- 洗腎病人
- 準備做器官或骨髓移植的病人
當患者被篩檢出為潛伏性結核病患者(LLTBI)時才開始預防性治療,以避免不完整的療程導致多重抗藥性結核病(MDR-TB)。
References
- Taiwan Centers for Disease Control. Recommendations for postponing BCG vaccination. Available from: https://www.cdc.gov.tw/Uploads/f9ddf264-1994-41a0-bc8e-e120e422a9c6.pdf
- Taiwan Centers for Disease Control. (28 March 2019). IT’S TIME! At-risk groups urged to get LTBI screening as Taiwan continues campaign to end TB. Available from: https://www.cdc.gov.tw/En/Bulletin/Detail/cHsOe6RCP5mIwPAOJRDeDw?typeid=158
- Chen YY, Pan SW, Shen HS, Chuang FY, Feng JY, Su WJ. Declining trend in incidence of tuberculosis in adolescents and young adults in Taiwan. Eur Respir J. 2019 May 2;53(5):1801305. doi: 10.1183/13993003.01305-2018. PMID: 31048371.
- Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019 Sep 12;54(3):1900655. doi: 10.1183/13993003.00655-2019. PMID: 31221810.
- Centers for Disease Control and Prevention. (2018). Deciding when to treat latent TB Infection. Available from: https://www.cdc.gov/tb/topic/treatment/decideltbi.htm
- 衛生福利部疾病管制署. (2015). 105年1月1日起卡介苗接種年齡調整為出生滿5個月,建議接種年齡出生滿5-8個月. https://www.cdc.gov.tw/Category/ListContent/3263Cslsb96HH82yOx-T8A?uaid=kxpu7SYN8j_1_-mUMds9Ug
- Spicer KB, Turner J, Wang SH, Koranyi K, Powell DA. Tuberculin skin testing and T-SPOT.TB in internationally adopted children. Pediatric Infectious Diseases J. 2015 Jun;34(6):599-603. doi: 10.1097/INF.0000000000000680. PMID: 25973937.
- 衛生福利部疾病管制署. (2017). 結核病診治指引, 第六版. https://www.cdc.gov.tw/File/Get/FQWRAW1kzwxQSzQKSXj5iw
為何選擇T-SPOT®.TB
T-SPOT.TB:小小一管,大大精準¹
T-SPOT.TB是由診斷業界翹楚 Oxford Immunotec, 研發的結核病單次驗血檢測,特別設計以降低分析變異,同時盡可能提高靈敏度,即使針對免疫缺陷病患也是如此²。
IGRA科技利用的是個別效應T細胞(包含CD4+與CD8+)受結核病特定抗原激發後,所分泌釋放的丙型干擾素。T-SPOT.TB檢測使用酶聯免疫斑點法(ELISPOT)來找出受結核桿菌激發的T細胞,原理是捕捉T細胞附近所分泌的丙型干擾素。
檢測中含括的T細胞數目會影響結核病感染的判讀標準。T-SPOT.TB檢測與其他檢測不同之處,在於每次均使用相同數量,也就是每孔中都250,000個細胞,每次檢測使用孔1,總計高達1,000,000個細胞,片刻間幫您測定是否感染結核病。
T-SPOT.TB檢測與其他LTBI檢測的比較
深入了解T-SPOT.TB檢測
更多資訊T-SPOT.TB檢測的好處

-
對不同族群一樣精準1
- 免疫抑制患者
- 卡介苗接種者
-
是美國食藥署核准給2歲以上兒童使用唯一的IGRA檢測2
-
穩定一致的結果3
- 靈敏度:98.8%
- 特異度:99.1%
-
針對法定臨界值附近的結果有高檢測精準度,有效防止不當治療3, 4。
自動化T-SPOT.TB檢測 3, 5
T-SPOT.TB檢測可使用經CE認證之T-Cell Select™試劑套組進行自動化,讓工作流程更為流暢、簡化物流、並節省技術人員寶貴的時間。
2歲以上均適用
T-SPOT.TB是美國食藥署核准給2歲兒童以上使用唯一的IGRA檢測1
眾所周知,適用於成人的診斷檢測,在兒童身上並不一定有相同的準確度。可幸的是,T-SPOT.TB檢測不僅適用於健康兒童,也適用於患有糖尿病或免疫缺陷的兒童。

與我們聯絡
與我們聯絡
Taiwan
正茂生物科技股份有限公司
114-94 台北市內湖區新湖一路145號6樓
Email: sales@genmall.com.tw
Phone: 886-2-27960803
FAX: 886-2-27310833
Singapore
Oxford Immunotec Limited (Singapore Branch)
160 Robinson Road #23-03
SBF Center, Singapore 068914
Email: enquiry-RoA@oxfordimmunotec.com
Phone: +65 6246 6861
References
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-V3. Abingdon, UK. September 2020.
- Oxford Immunotec. T-SPOT.TB Package Insert TB-PI-US-0001 V9. Abingdon, UK. February 2021.
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-v3. Abingdon, UK. 2016.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, IGRA Expert Committee, Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010; 59(RR-5:1-25.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American ThoracicSociety/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. Published online December 8, 2016:ciw694. doi:10.1093/cid/ciw694
