T-SPOT®.Covid

Complete your
view of COVID-19
with T cell testing.
Use T-SPOT.COVID to reliably assess
the immune response to SARS-CoV-2
Serology testing isn’t enough to understand your immune system.
The T-SPOT.COVID test is a simple blood test intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, specifically the T cell response. The test uses the T-SPOT Technology, a modified ELISPOT technology, with an optimised antigen mix, based on SARS-CoV-2 structural proteins, spike and nucleocapsid (S and N), and allows the maximum breadth of the immune response to be measured.
The T-SPOT.COVID test1
CE marked for IVD use
- Proven to detect evidence of a T cell-mediated immune response to SARS-CoV-2 infection in PCR positive patients with a negative serology test result
- Results, complemented by serology results, give a comprehensive view of an individuals’ immune response to SARS-CoV-2
- Results can be generated quickly, the laboratory process takes just 2 days
Research Use Only (RUO)
- Interrogates the immune system’s T cell response to enable research into the measurement of the strength of that response to SARS-CoV-2.
- Addresses challenges in T cell measurement using a standardized and proprietary platform, T-SPOT that has been in used since 2002, developed based on the ELISPOT methodology.
- Ability to test samples up to 32 hours after blood collection without the need for cryopreservation.
- Completes picture of our protective immunity going beyond investigating humoral immune response to include cell-mediated immune response.
Product Code: COVID.435/300 RUO
Product Description: COVID.435/300 Strip Kit
Why partner with Oxford Immunotec?
A leading global diagnostics company with an 18-year history of transforming T cell science into meaningful insights using the T-SPOT Technology. The T-SPOT Technology is proven in TB diagnostics with > 20 million tests shipped to > 50 countries where the T-SPOT.TB test is approved for clinical use.
_Why offer COVID-19 T cell testing?_
Performance of the T-SPOT.COVID test
| Time after positive PCR results | T-SPOT.COVID positive agreement | T-SPOT.COVID negative agreement |
| < 60 days | 96.6% (84/87) | – |
| > 60 days | 83.3% (40/48) | – |
| N/A | – | 98.0% (96/98) |
- Positive agreement: 135 individuals with a previous positive COVID-19 test result
- Negative agreement: 98 individuals at low risk of acquiring infection and who have never had a positive COVID-19 test result
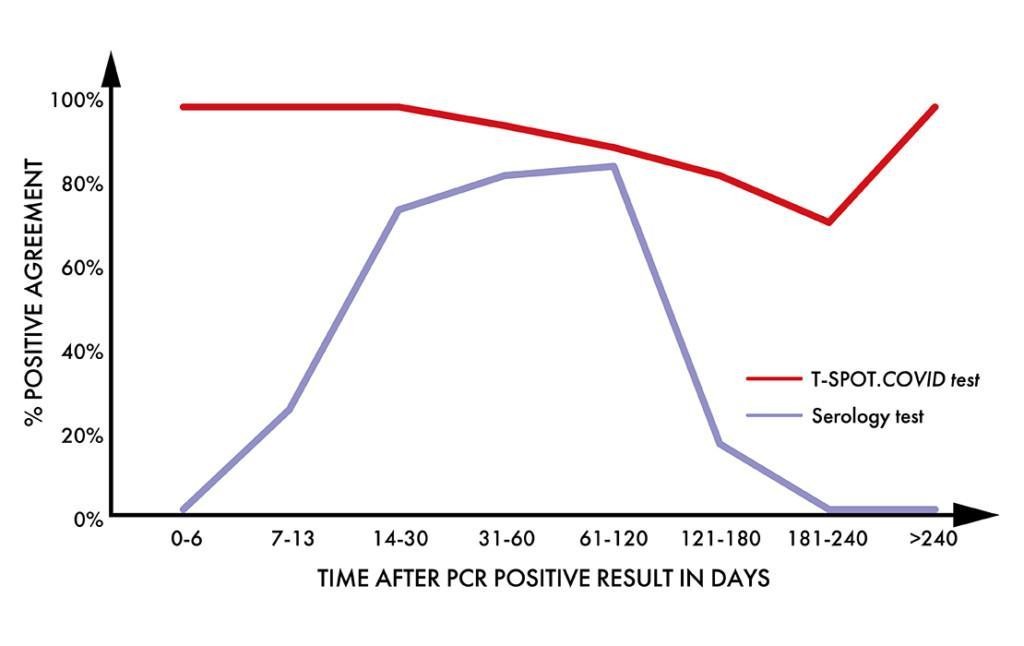
Higher positive agreement than a leading serology test1
In a study of 135 individuals, with a previous positive PCR result for SARS-CoV-2 infection, samples were analysed by both the T-SPOT.COVID test and a leading serology test.
- T cell positive agreement remained high throughout the length of the study
- Serology positive agreement decreased over time
- Serology positive agreement was low before 14 days
Serology alone is not enough1
Research shows T cell response and antibody immune response are independently correlated to protection against SARS-CoV-2. Antibody response represents half of the immune system and T cells are the other half.
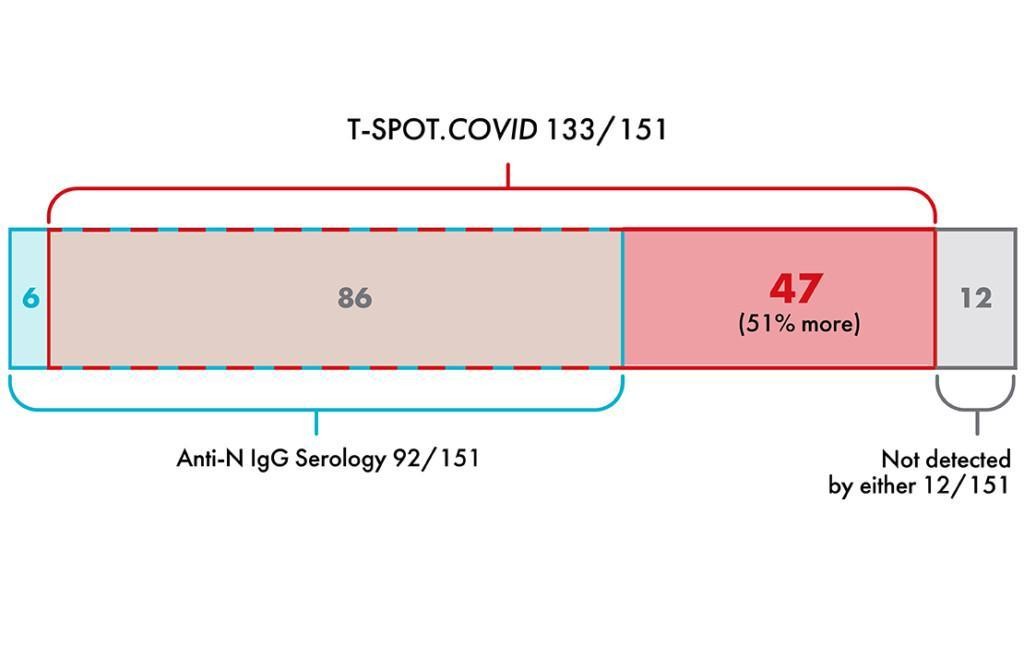
Further analysis of the study cohort evaluated overlap between the two test methods.
The T-SPOT.COVID test identified 51% more PCR positive individuals than serology testing alone
How to access T-SPOT.COVID
CONTACT US_FAQ_
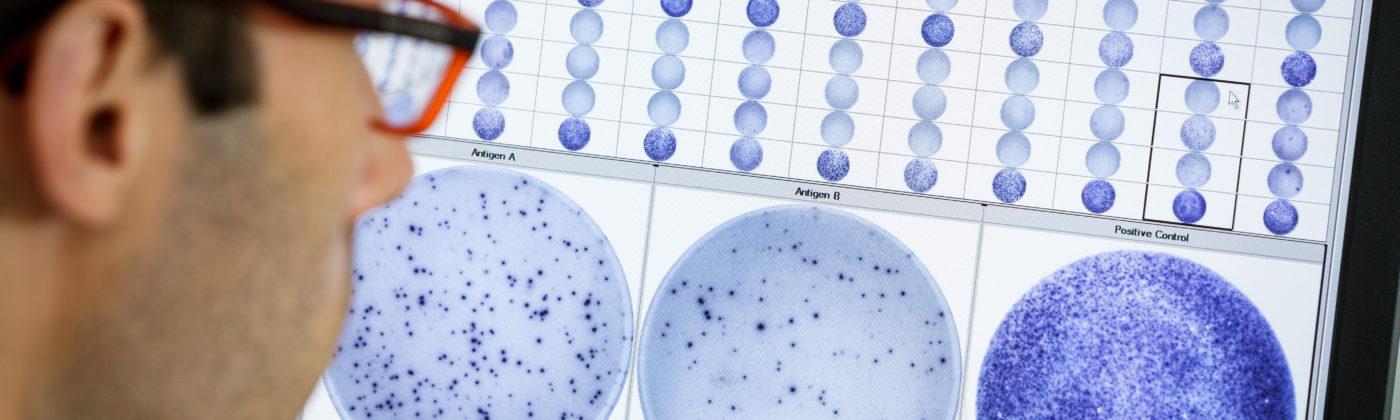
- How does the T-SPOT technology work?
Our T-SPOT.COVID test based on ELISPOT technology is normalised for both cell number and culture conditions. This means that the test standardises the number of cells and removes serum factors that could adversely affect results, making it the most sensitive and specific test for T cell measurement. A blood sample is collected using routine phlebotomy and a standard blood collection tube from which a subset of white blood cells, known as peripheral blood mononuclear cells (PBMCs), are isolated. The cells are washed, counted and normalised to create a standard cell suspension. A standard number of cells are added into specially designed plates and stimulated with antigens specific to the disease under study. Cells responding to these antigens release a chemical messenger known as a cytokine. Cytokine antibodies are used to directly capture the cytokine as it is released by the cells. A secondary labelled antibody is added and binds to the captured cytokine. A detection reagent is added and reacts with the secondary labelled antibody. This reaction produces spots, which are a footprint of where the cytokine was released. Spots are then enumerated. - What does a positive T-SPOT.COVID test result mean?
A positive test result means that the patient has T cells that are reactive to the SARS-CoV-2 specific peptides used in the T-SPOT.COVID test. It is highly likely that they have been exposed to the SARS-CoV-2 virus. - What does a negative test result mean?
A negative test result means that the patient does not have T cells that are reactive to the SARS-CoV-2 specific peptides used in the test. It is therefore unlikely that they have been exposed to the SARS-CoV-2 virus. - What regulatory approval does the T-SPOT.COVID test have?
The T-SPOT.COVID test is CE marked and has been submitted to the FDA for Emergency use authorisation.
T cells in COVID-19
Helen Fletcher, Professor of Immunology at the London School of Hygiene and Tropical Medicine, talks about the importance of T cells in COVID-19 and answers some topical questions, including:
- What role might T cells have in COVID-19?
- Are antibodies or T cells more important to COVID-19?
- How might T cells be used for testing?
Public Health England2
A prospective cohort study performed by Public Health England used the T-SPOT research use only platform to provide detailed information on SARS-CoV-2-specific T cells in immune responses to SARS-CoV-2 infection. In this study, 2,826 individuals identified as keyworkers were tested for anti-spike IgG and SARS-CoV-2 responsive T cells.
Key findings
- T cell responses could be detected in individuals who had tested positive for SARS-CoV-2 using PCR, but were seronegative
- T cells may play a role in protection against re-infection with the SARS-CoV-2 virus, even in the absence of detectible antibody