Automating the T-SPOT.TB test

In TB testing, why trade accuracy for efficiency when you don’t have to?
The T-SPOT.TB test involves multiple steps which help to ensure accuracy when reporting latent tuberculosis (TB) results; but it no longer has to be a fully manual procedure. By choosing to automate just one, a number, or all of the processes within the workflow, your lab can select the most cost-effective solution for your specific needs and offer this clinically superior IGRA.1,2
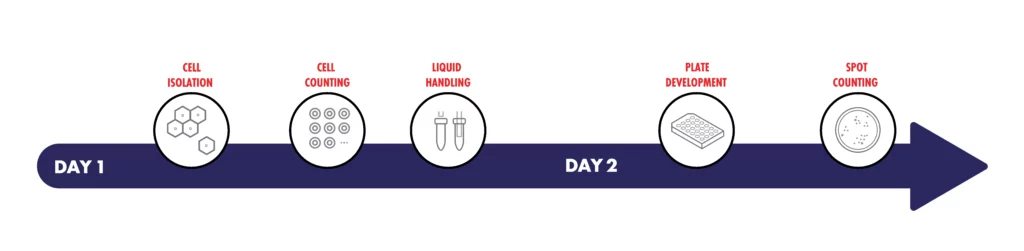
What makes each workflow step important?
| Cell Isolation | Enriches the desired population of peripheral blood mononuclear cells (PBMC) from whole blood collected using routine phlebotomy |
| Cell Counting | Facilitates correction for variation in PBMC counts and ensures the required number of cells are used to produce a reportable result |
| Liquid Handling | Essential for the transfer of samples and reagent solutions throughout the workflow for accurate test results |
| Plate Development | Interferon gamma antibodies capture a cell specific response to TB-specific antigens |
| Spot Counting | Single cell resolution for the detection of interferon gamma releas |
The isolation of PBMCs from whole blood is critical
What is T-Cell Select™?
The cell isolation step of the T-SPOT.TB workflow is now automated using the T-Cell Select reagent kit eliminating manual errors and reducing technician time. This PBMC isolation reagent uses positive selection of targeted immune cells with magnetic bead-based cell separation to simplify the preparation of cells for the T-SPOT.TB test.
The T-SPOT.TB test with the T-Cell Select reagent kit provides you with:
- Superior clinical performance 1,2
- Reduced hands-on time
- Extended blood sample stability
- Improved blood sample logistics
- Maximized operational efficiency
- Increased sample throughput potential
Speak with an expert about improving your TB testing
CONTACT USDon’t rely on processes outside your lab.
Take control with improved blood sample stability and logistics.
Samples stored at room temperature for 54 hours
Enables easy centralization of sample processing and further simplifies sample logistics.
- Flexibility for sample batching
- Simplify sample delivery
- Extend sample delivery
- Expand the reach of your laboratory
Simple sample logistics using routine phlebotomy
Ensure all up-front processing of the specimen after blood draw is done in a laboratory setting where controlled conditions are leveraged to mitigate the impact of confounding variables.
| Sources of IGRA variability3,4 | T-SPOT.TB | Other IGRAs |
|---|---|---|
| Specialized tubes | No | Yes |
| On-site refrigeration | No | Yes |
| On-site incubation | No | Yes |
| Shaking of blood tubes | No | Yes |
| Blood volume variability | No | Yes |